ELabELN for Pharmaceuticals: FDA-Compliant Digital Lab Notebooks for Drug Discovery
Stop losing critical research data to paper notebooks and spreadsheets scattered across your pharma lab. ELabELN delivers free, FDA 21 CFR Part 11-compliant electronic lab notebooks purpose-built for drug discovery, formulation development, and pharmaceutical testing workflows. Start with our Community Edition at zero cost and scale seamlessly as your research grows, ensuring data integrity, audit readiness, and collaboration without the enterprise price tag or complexity that slows down science.


ELN Solutions That Accelerate Pharma Research and Ensure Regulatory Compliance
Pharmaceutical researchers face unique pressures: regulatory audits demand flawless documentation, drug development timelines are unforgiving, and a single data integrity issue can jeopardize years of work. Paper notebooks create audit nightmares, version confusion destroys collaboration between chemistry and biology teams, and manual transcription introduces errors that compromise patent applications and regulatory submissions. ELabELN eliminates these risks with automated audit trails, encrypted data storage, and electronic signatures that meet FDA requirements without requiring IT expertise or months of validation.
Our platform adapts to every pharma workflow from early discovery to late-stage development. Document synthesis protocols with automated timestamps, track compound testing with built-in inventory management, collaborate across global research sites with real-time access controls, and generate audit-ready reports in minutes instead of days. Start with the free Community Edition for unlimited users per team, then upgrade to ELN Suite when you're ready for advanced analytics, full validation support, custom integrations, and priority technical support. Your goal is developing life-saving therapies — let ELabELN handle the documentation complexity while you focus on the science.
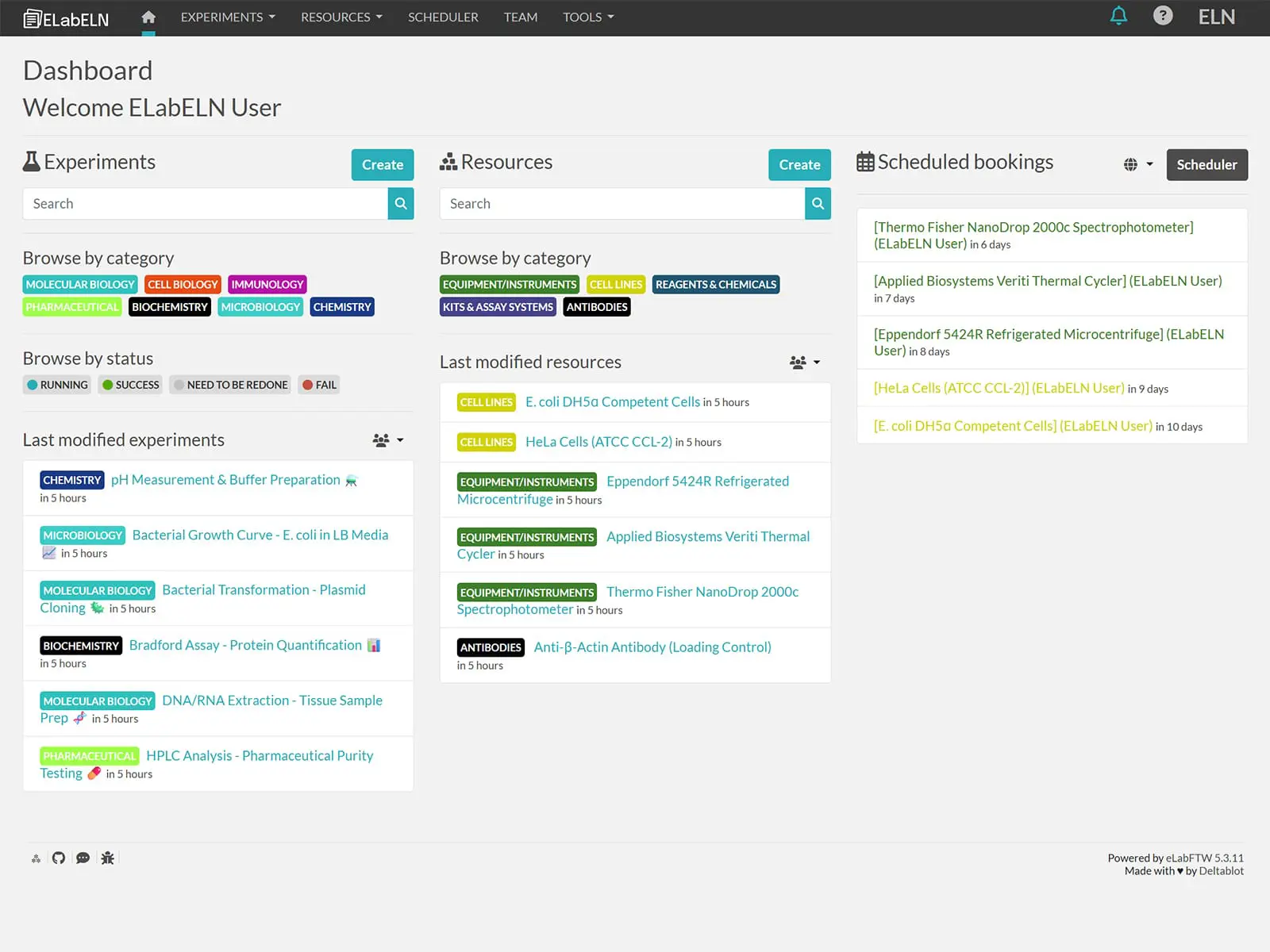
Stop risking regulatory delays and data loss with outdated paper systems. Discover pharmaceutical ELN solutions built for compliance and speed.
Comprehensive ELN Features for Every Pharma Workflow and Regulatory Need
Pharmaceutical research demands documentation tools that satisfy both scientific rigor and regulatory scrutiny. ELabELN provides specialized features covering synthesis documentation, analytical testing, formulation development, and compliance reporting, all designed to accelerate drug discovery while maintaining the data integrity standards required for FDA submissions, patent applications, and regulatory audits.
Core Documentation & Compliance Tools
FDA 21 CFR Part 11 Electronic Signatures
Legally binding electronic signatures with complete audit trails meet FDA requirements for electronic records and regulatory inspections.
Automated Audit Trail Tracking
Every edit, view, and signature is permanently recorded with user identification and timestamps for regulatory submissions.
Synthesis Protocol Templates
Pre-built templates for synthetic chemistry workflows capture reaction conditions, reagent details, and yield calculations automatically.
Data Management & Search Capabilities
Compound Structure Search
Find experiments by chemical structure, molecular formula, or compound ID instantly across thousands of synthesis records.
Batch Record Management
Track manufacturing batches from initial synthesis through quality control testing with linked experiment records.
Advanced Filtering & Tagging
Organize experiments by project, therapeutic area, compound series, or development stage for rapid retrieval.
Collaboration & Team Workflows
Multi-Site Research Coordination
Enable chemistry teams, biology groups, and formulation scientists across global sites to access the same real-time data.
Structured Review Workflows
Route experiments through required review chains automatically with tracked approvals from PIs, QA, and regulatory affairs.
Cross-Functional Data Sharing
Grant controlled access to analytical chemists, toxicologists, and regulatory teams while maintaining IP protection.
Analytics & Regulatory Reporting
Automated Compliance Reports
Generate audit-ready documentation for FDA inspections, patent applications, and regulatory submissions with one click.
Cross-Experiment Analytics
Analyze structure-activity relationships across hundreds of compounds and identify optimization trends for data-driven decisions.
Custom Dashboard Creation
Build visual dashboards tracking project progress, compound advancement, and team productivity for leadership reporting.
Get Started Quickly with Pharmaceutical-Grade ELN at Zero Cost
"*" indicates required fields


